Advancing Rare Disease Care Through Genomic Medicine and Whole-Person Support
The 2nd TMU–SHH Rare Disease International Symposium on January 31st 2026 brought together international experts, clinicians, and researchers to share the latest advances in rare disease diagnosis, treatment, and patient-centered care. An event co-hosted by Compass Bioinformatics, we’re building on the success of its inaugural year, with the symposium reaffirming TMU–Shuang Ho Hospital’s long-term commitment to improving outcomes for rare disease patients and their families.
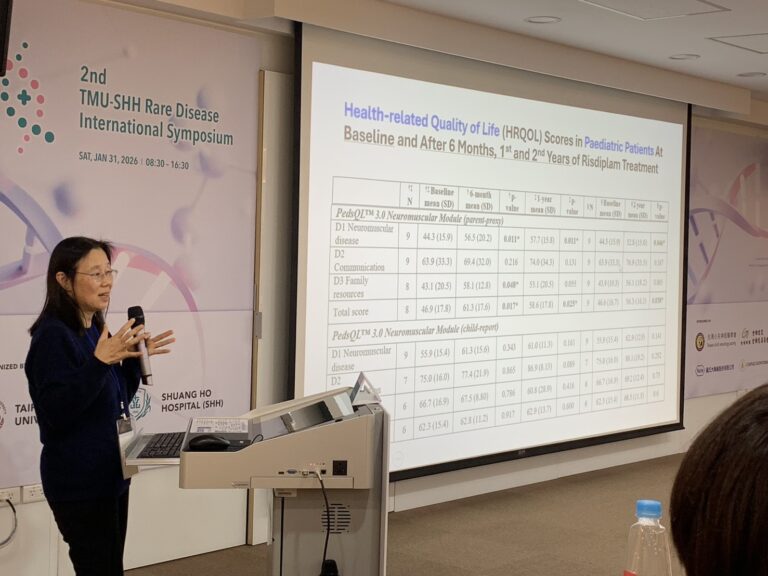
Translating Clinical Trial Data into Real-World Impact for SMA Patients
Long-Term Outcomes of Risdiplam Oral Therapy
The morning session opened with a keynote presentation by Professor Dr. Sophelia Chan, MD (HKU Med), who shared clinical trial monitoring data and real-world experience in spinal muscular atrophy (SMA) management.
Professor Chan presented long-term safety and efficacy data for Risdiplam, demonstrating stabilization of motor function over 36 months of treatment. She also discussed how Health-Related Quality of Life (HRQOL) scores are used to quantitatively assess patient outcomes.
From “Cold Numbers” to Lived Experiences
Beyond numerical endpoints, Professor Chan emphasized the importance of translating clinical metrics into real-life scenarios. Through patient stories, she illustrated how treatment benefits extend beyond motor scores to transform daily life for both pediatric patients and their families.
One particularly moving case described a child who had been confined to his room for most of his life, but was able to visit an aquarium to celebrate his birthday—an experience made possible through improved disease stability with oral therapy. These stories highlighted how quality of life improvements represent meaningful success in rare disease care.




Genomic Medicine in Pediatric Neurological Diseases: Lessons from Japan
Complex Genetic Disorders Beyond Traditional Diagnosis
The second keynote was delivered by Professor Dr. Toshiyuki Yamamoto, MD (Tokyo Women’s Medical University), who presented Japan’s experience in genomic medicine for pediatric neurological disorders through multiple published case studies.
His presentation included complex conditions such as:
- Microdeletion syndromes
- Dual deletions within a single patient
- Disorders caused by chromoanasynthesis
- Chromosomal translocations and other complex rearrangements
These cases underscored the limitations of symptom-based diagnosis and conventional genetic testing, particularly for patients with atypical or overlapping clinical features.
Precision Diagnosis Enabled by NGS and Long-Read Sequencing
Professor Yamamoto demonstrated how next-generation sequencing (NGS) and long-read sequencing technologies, including nanopore sequencing, have enabled precise diagnosis of cases that were previously undiagnosable.
Notably, one case revealed overlapping genomic deletions shared between a Japanese patient and a French patient, highlighting both the resolution of modern genomic tools and the importance of global data comparison in rare disease research.
During the Q&A session, Professor Yamamoto also noted that the integration of AI-based genetic analysis software has significantly reduced analysis time and streamlined variant interpretation workflows.
Never Miss What Matters: 98.6% Sensitivity to Find the Causative Variant.
Rare Diseases and Genetic Findings
| Disease/Syndrome | Associated Gene(s) or Region | Takeaway Notes |
| Gorlin Syndrome | PTCH1 | Identified via a very small deletion that was invisible to standard G-banding but caught by microarray. |
| 22q11.2 Microdeletion | Chromosome 22q11.2 | A classical microdeletion syndrome with characteristic heart defects and facial features. |
| Williams Syndrome | Region-specific | Mentioned as a classical syndrome with a “diagnostic yield” that is easy to reach via clinical findings. |
| Prader-Willi Syndrome | Chromosome 15 | Traditionally a microdeletion; transcript notes a rare “dual syndrome” case where the patient also had Sotos. |
| Sotos Syndrome | Chromosome 5 | A classical microdeletion syndrome; can coexist with other deletions (e.g., in dual syndrome cases). |
| Smith-Magenis Syndrome | 17p11.2 (Deletion) | Characteristic findings; results from Non-Allelic Homologous Recombination (NAHR). |
| Potocki-Lupski Syndrome | 17p11.2 (Duplication) | The “reciprocal” of Smith-Magenis; harder to diagnose clinically as it lacks distinctive facial findings. |
| 5q31.3 Microdeletion Syndrome | PURA | A “newly identified” syndrome established via the DECIPHER database; involves seizures and myelination delay. |
| Gitelman-like symptoms (implied) | SLC12A3 | A paternal deletion unmasked a maternal mutation, leading to homozygous-like symptoms. |
| Pelizaeus-Merzbacher-like Disease | GJC2 | Caused by Uniparental Disomy (UPD) from the mother, leading to white matter abnormalities. |
| Infantile Spasms | KCTD3 | A homozygous mutation created by “monosomy rescue” (paternal Uniparental Disomy). |
| Epileptic Encephalopathy | MEF2C | Caused by a “position effect” where a translocation far from the gene still disrupted its expression. |
| Intractable Epilepsy | FBXW7 | Another “position effect” case; translocation caused decreased expression of the gene. |
| Complex Structural Abnormality | Chromosome 21 | A “Down-like” appearance caused by Chromoanasynthesis (chromosome shattered into 7 pieces). |
From First-Tier Testing to Comprehensive Genomic Analysis
Clinical Take-Home Messages for Pediatric Neurology
Professor Yamamoto summarized several key clinical insights:
- Chromosomal microarray analysis (CMA) remains the first-tier test for children with neurological symptoms of unknown etiology.
- Comprehensive genomic analysis is essential to uncover the full genetic background of complex cases.
- Copy-neutral rearrangements and very small copy number changes may be misdiagnosed as disease-causing variants.
- Long-read sequencing offers a powerful solution for resolving these diagnostic blind spots, as demonstrated by the presented case studies.
TMU–SHH’s Vision for Rare Disease Care: Whole-Person Support
Building a Comprehensive Rare Disease Care Ecosystem
Now in its second year, the TMU–SHH Rare Disease Symposium reflects a broader institutional mission: to enhance care and prevention for rare disease patients, raise awareness among patients, families, and the community, and reduce the long-term psychosocial impact of rare disease diagnosis.
This mission aligns with TMU–SHH’s goal of Whole-Person Totality Care (全人照護服務)—integrating medical treatment, social resources, and community support so that patients and families are not alone in their medical journey.
Long-Term Leadership in Pediatric Rare Disease Care
Under the leadership of Dr. Yung-Ting Kuo, Director of the Department of Pediatrics, TMU–SHH has cultivated a robust rare disease care framework for more than 20 years, emphasizing continuity of care, family support, and interdisciplinary collaboration.
Clinical Insights from SHH Pediatrics: Rare Disease Case Distribution
Understanding the Rare Disease Landscape at SHH
SHH Pediatrics also shared institutional data showing that three diseases—Carnitine Deficiency, Duchenne Muscular Dystrophy, and Myotubular Myopathy—account for nearly 50% of all rare disease cases treated at the hospital.
An additional 24% consists of 11 distinct rare diseases, highlighting the diversity of conditions encountered in clinical practice.
Implications for Care and Diagnosis
This distribution underscores the need to:
- Develop specialized expertise and standardized care pathways for higher-prevalence rare diseases
- Maintain broad diagnostic capabilities, including advanced genomic tools, to support patients with ultra-rare and complex conditions
Moving Forward: Integrating Genomics, AI, and Compassionate Care
The morning sessions of the 2nd TMU–SHH Rare Disease International Symposium demonstrated how precision medicine, long-read sequencing, AI-assisted analysis, and patient-centered care are collectively reshaping the future of rare disease diagnosis and management.
Through continued interdisciplinary collaboration and international knowledge exchange, TMU–SHH remains committed to advancing rare disease care—combining scientific innovation with compassionate, whole-person support.


